In order to affix the necessary CE marking the manufacturer must document that the medical device bearing his name complies with the essential requirements of Annex I of the relevant legislation. Depending on the product type, the appropriate Conformity Assessment Procedure must be followed, as shown in the table below.
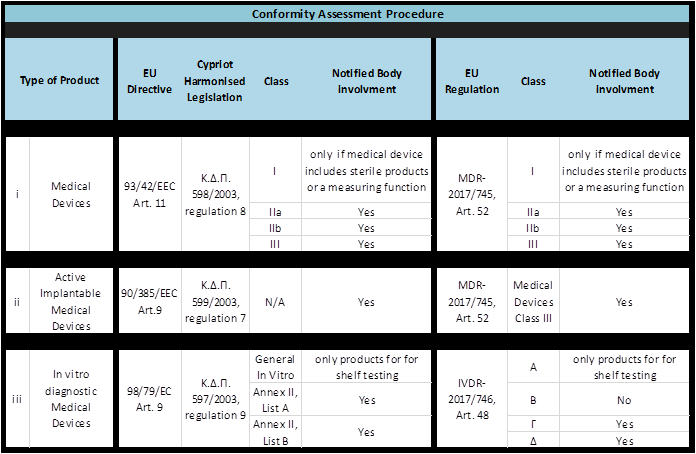
Where the Conformity Assessment Procedure requires the intervention of a notified body, the manufacturer may apply to a body of his choice in the context of the tasks for which that body has been notified. For the list of Notified Organizations click here.
Compliance of a product with the relevant legislation may be presumed to some extent by conforming to the relevant harmonized European standards (eg EN ISO 13485: 2016). Click here for the list of Harmonized Standards.
Once the medical device's compliance with the essential requirements of the legislation has been documented, the manufacturer shall affix the CE marking to the product and draw up a Declaration of Conformity with the essential requirements. Where a Notified Body is involved in the Conformity Assessment Process, the CE marking shall be accompanied by the four-digit identification number of that Organization.
The European Commission’s website (Your Europe) has more information on CE marking.




Last Update:
16/10/2020 11:08:58